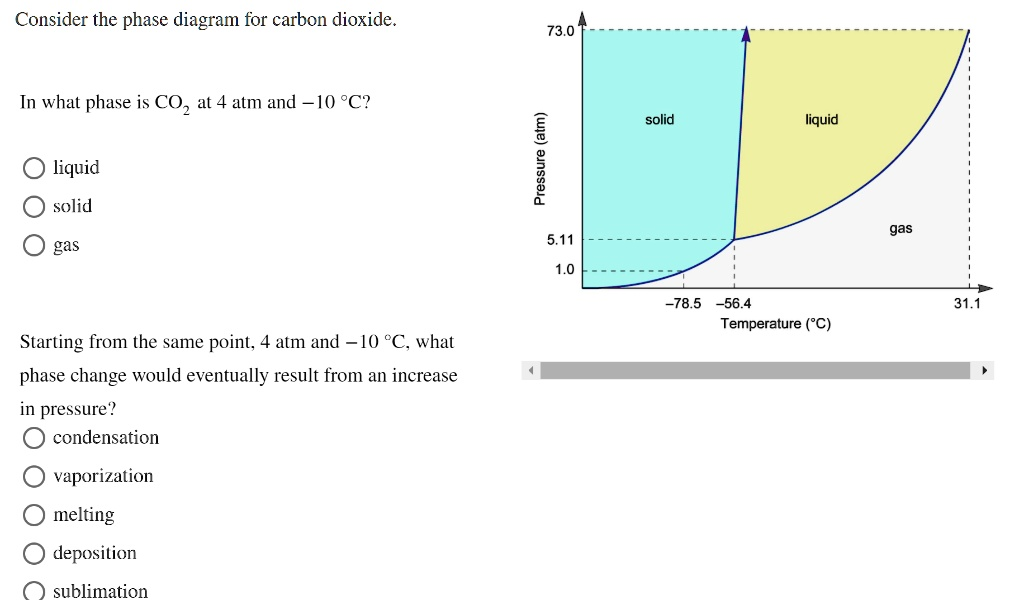
Consider the phase diagram for carbon dioxide. In what phase is CO2 at 4 atm and -10 °C? – Solid – Liquid – Gas Starting from the same point; 1 atm and 0 °C, what phase change would eventually result from an increase in pressure? – Condensation – Vaporization – Melting – Deposition – Sublimation
The Correct Answer and Explanation is:
Here are the step-by-step solutions to the questions based on the provided phase diagram for carbon dioxide.
Question 1: In what phase is CO₂ at 4 atm and -10 °C?
- Locate the point: Find the temperature -10 °C on the horizontal axis (Temperature). This is to the right of -56.4 °C.
- Find the pressure: Locate the pressure 4 atm on the vertical axis (Pressure). This is between the marked points 1.0 atm and 5.11 atm.
- Identify the phase: Trace a vertical line up from -10 °C and a horizontal line across from 4 atm. The point where they intersect falls within the region labeled gas.
Answer: The correct option is gas.
Question 2: Starting from the same point, 4 atm and -10 °C, what phase change would eventually result from an increase in pressure?
- Identify the starting point: As determined above, the point (4 atm, -10 °C) is in the gas phase.
- Understand the change: “An increase in pressure” at a constant temperature means moving straight up vertically on the diagram from your starting point.
- Observe the transition: As you move vertically up from the point (4 atm, -10 °C), you will cross the boundary line separating the gas phase from the liquid phase.
- Name the phase change: The transition from a gas to a liquid is called condensation.
Answer: The correct option is condensation
