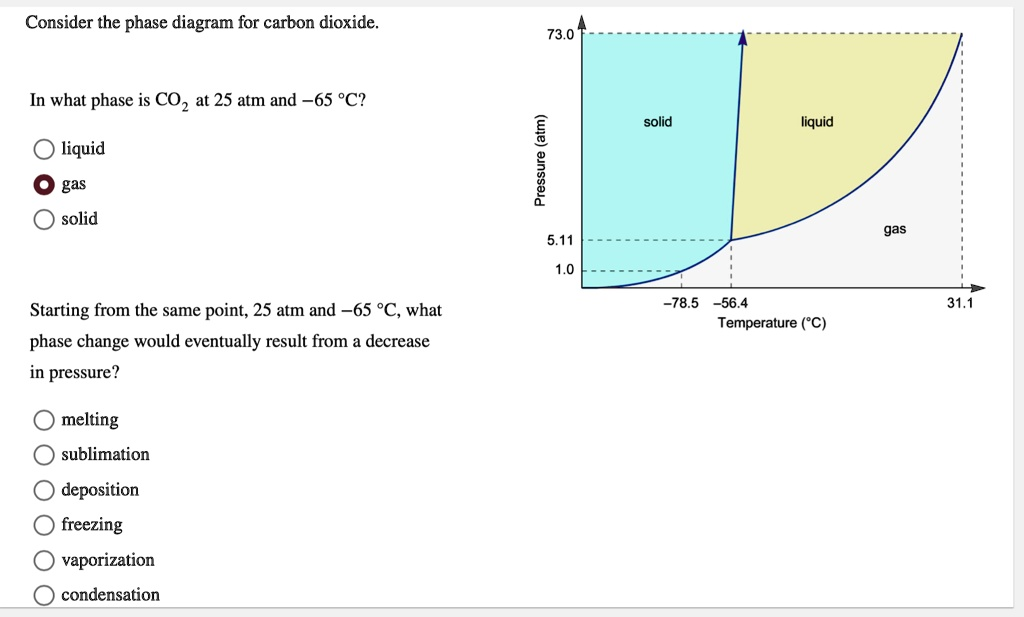
COz 25 atm and -65 PC? solid liquid 0 L 5.11 1.0 liquid gas solid gas Starting from the same point; 25 atm and ~65 ‘C, what phase change would eventually result from a decrease in pressure? -78.5 556.4 Temperature (‘C) 31,1 melting sublimation deposition freezing vaporization condensation
Consider the phase diagram for carbon dioxide: 73.0 In what phase is COz 25 atm and -65 PC? solid liquid 0 L 5.11 1.0 liquid gas solid gas Starting from the same point; 25 atm and ~65 ‘C, what phase change would eventually result from a decrease in pressure? -78.5 556.4 Temperature (‘C) 31,1 melting sublimation deposition freezing vaporization condensation
The Correct Answer and Explanation is:
Here are the correct answers and a detailed explanation based on the provided phase diagram for carbon dioxide.
First question: In what phase is CO₂ at 25 atm and –65 °C?
Correct Answer: solid
Second question: Starting from the same point, 25 atm and –65 °C, what phase change would eventually result from a decrease in pressure?
Correct Answer: sublimation
Explanation
Part 1: Determining the Initial Phase
To find the phase of carbon dioxide (CO₂) at a specific pressure and temperature, you must locate that point on the phase diagram. The diagram shows pressure on the vertical (y) axis and temperature on the horizontal (x) axis.
- Locate the temperature: Find –65 °C on the x-axis. This temperature is to the left of the triple point temperature (–56.4 °C) but to the right of the normal sublimation point (–78.5 °C).
- Locate the pressure: Find 25 atm on the y-axis. This pressure is well above the triple point pressure (5.11 atm) and below the critical pressure (73.0 atm).
- Find the intersection: Trace a vertical line upwards from –65 °C and a horizontal line across from 25 atm. The point where these two lines intersect falls squarely within the region labeled solid (the blue-shaded area). Therefore, at 25 atm and –65 °C, CO₂ is in the solid phase.
Part 2: Identifying the Phase Change
The second question describes a process starting from the point identified above (solid CO₂ at 25 atm and –65 °C) and then decreasing the pressure while the temperature remains constant at –65 °C.
- Trace the process on the diagram: A decrease in pressure at a constant temperature is represented by moving vertically downwards from the starting point on the phase diagram.
- Identify the phase transition: Starting at (–65 °C, 25 atm) in the solid region, moving straight down means crossing the boundary line that separates the solid phase from the gas phase.
- Name the phase change: The direct transition from a solid to a gas is known as sublimation. Because the temperature (–65 °C) is below the triple point temperature (–56.4 °C), the CO₂ bypasses the liquid phase entirely. If the temperature were above –56.4 °C, a decrease in pressure might cause melting (solid to liquid) followed by vaporization (liquid to gas). However, at –65 °C, only sublimation is possible as the pressure is lowered.
