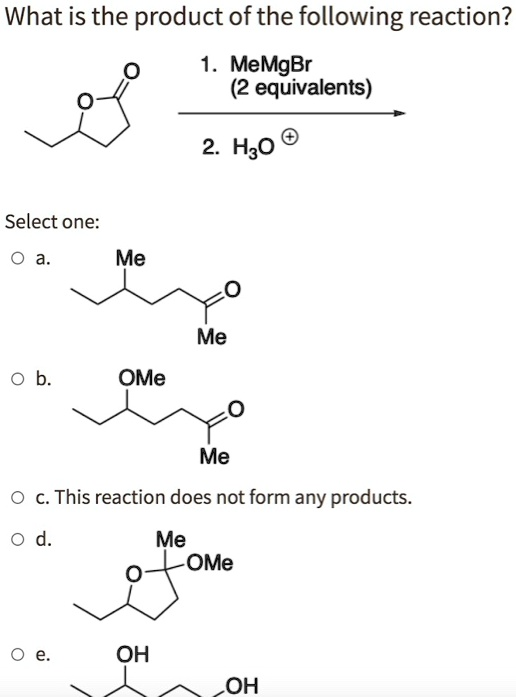
What is the product of the following reaction? MeMgBr (2 equivalents) 2 H3O Select one: Me Me OMe Me c. This reaction does not form any products: Me OMe OH OH
The Correct Answer and Explanation is:
The reaction involves two equivalents of methylmagnesium bromide (MeMgBr) reacting with a carbonyl-containing compound. The key points are as follows:
- MeMgBr (Grignard reagent): This is a strong nucleophile that adds to carbonyl groups in a nucleophilic addition reaction.
- H3O+: After the nucleophilic addition, the reaction is typically quenched with an aqueous acid (H3O+) to protonate the intermediate and stabilize the product.
Given that there are two equivalents of MeMgBr, this means two nucleophilic additions occur. The reaction likely involves an ester or ketone as the starting material. The carbonyl group will be attacked by the MeMgBr twice, forming a product where two methyl groups (Me) are added to the original carbonyl carbon.
Now, based on the reaction, the product is most likely a tertiary alcohol where two methyl groups are attached to the same carbon center.
From the options given, the correct choice would be:
- e. OH OH
This corresponds to a product where two methyl groups are attached to the carbon that was originally part of the carbonyl group (likely a ketone), forming a tertiary alcohol after the reaction with H3O+.
So, the correct answer is e. OH OH.
