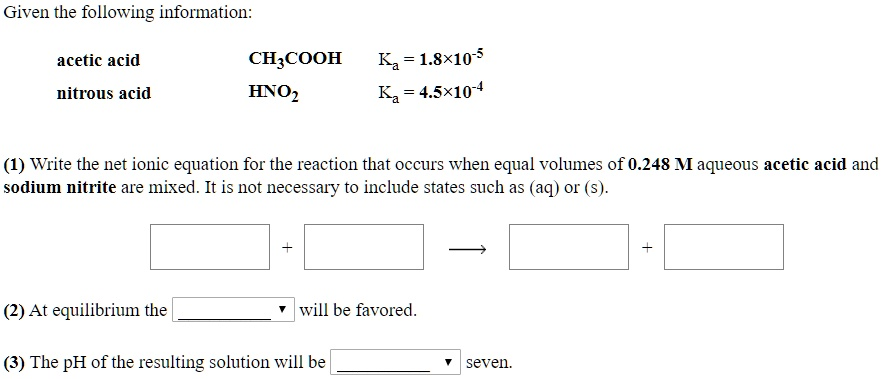
Write the net ionic equation for the reaction that occurs when equal volumes of 0.248 M aqueous acetic acid and sodium nitrite are mixed.
The Correct Answer and Explanation is:
ere are the solutions to the chemistry problem.
Correct Answer:
(1) Net Ionic Equation:
CH₃COOH + NO₂⁻ → CH₃COO⁻ + HNO₂
(2) At equilibrium the reactants will be favored.
(3) The pH of the resulting solution will be less than seven.
Explanation:
(1) Net Ionic Equation
The problem describes mixing aqueous acetic acid (CH₃COOH) and aqueous sodium nitrite (NaNO₂).
- Acetic acid (CH₃COOH) is a weak acid, so it does not dissociate completely in water and is written in its molecular form in a net ionic equation.
- Sodium nitrite (NaNO₂) is a soluble ionic salt that dissociates completely into sodium ions (Na⁺) and nitrite ions (NO₂⁻). The sodium ion (Na⁺) is a spectator ion, as it does not participate in the acid-base reaction.
- The nitrite ion (NO₂⁻) is the conjugate base of the weak acid nitrous acid (HNO₂), so it will act as a base.
The reaction is an acid-base reaction where the acid (CH₃COOH) donates a proton (H⁺) to the base (NO₂⁻). This forms the conjugate base of acetic acid, the acetate ion (CH₃COO⁻), and the conjugate acid of the nitrite ion, nitrous acid (HNO₂). The resulting net ionic equation is:
CH₃COOH + NO₂⁻ → CH₃COO⁻ + HNO₂
(2) Equilibrium Position
Acid-base equilibria always favor the side with the weaker acid and weaker base. To determine which side is favored, we compare the strengths of the two acids involved: acetic acid (reactants) and nitrous acid (products).
- Ka for acetic acid (CH₃COOH) = 1.8 × 10⁻⁵
- Ka for nitrous acid (HNO₂) = 4.5 × 10⁻⁴
Since the Ka of acetic acid (1.8 × 10⁻⁵) is smaller than the Ka of nitrous acid (4.5 × 10⁻⁴), acetic acid is the weaker acid. Because the equilibrium favors the formation of the weaker species, the reaction will favor the side with acetic acid. Therefore, the reactants will be favored.
(3) pH of the Solution
To determine the pH of the final solution, we must compare the strength of the initial acid (CH₃COOH) with the strength of the initial base (NO₂⁻).
- The acid strength is given by its Ka: Ka(CH₃COOH) = 1.8 × 10⁻⁵.
- The base strength of the nitrite ion (NO₂⁻) is given by its Kb. We can calculate Kb using the Ka of its conjugate acid (HNO₂) and the ion-product constant for water (Kw = 1.0 × 10⁻¹⁴):
Kb(NO₂⁻) = Kw / Ka(HNO₂) = (1.0 × 10⁻¹⁴) / (4.5 × 10⁻⁴) ≈ 2.2 × 10⁻¹¹
Now, we compare the Ka of the acid to the Kb of the base:
Ka(CH₃COOH) = 1.8 × 10⁻⁵
Kb(NO₂⁻) = 2.2 × 10⁻¹¹
Since Ka is much larger than Kb (1.8 × 10⁻⁵ > 2.2 × 10⁻¹¹), the acidic properties of acetic acid are stronger than the basic properties of the nitrite ion. This means the solution will be acidic, and the pH will be less than seven.
