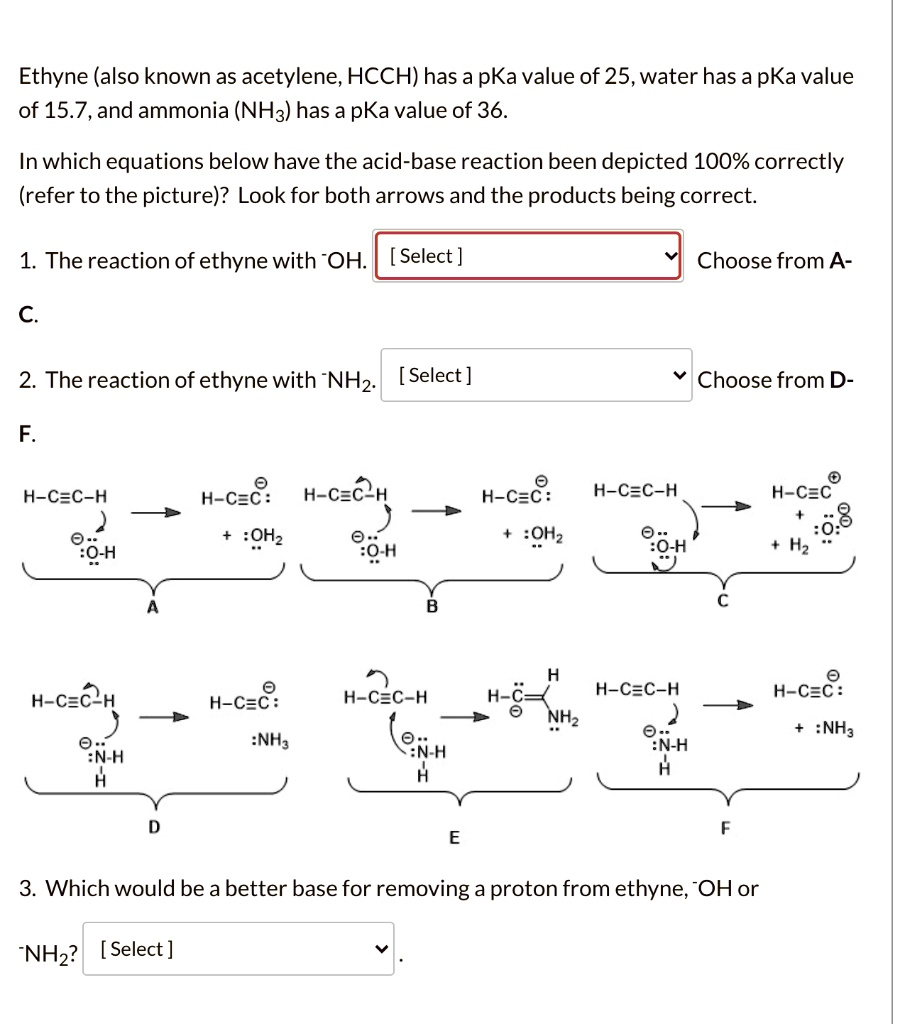
Ethyne (also known as acetylene; HCCH) has a pKa value of 25, water has a pKa value of 15.7, and ammonia (NH3) has a pKa value of 36. In which equations below have the acid-base reaction been depicted 100% correctly (refer to the picture)? Look for both arrows and the products being correct. The reaction of ethyne with OH: [Select] Choose from A- 2. The reaction of ethyne with NH3: [Select] Choose from D- F H-C≡C-H H-C≡C: H-C≡C H H-C≡C: H-C≡C-H H-C≡C: :NH3 H-C≡C-H H-C_ H-C≡C-H NH3 XN-H H-C≡C: :NH3 :NH3 PNH3 Which would be a better base for removing a proton from ethyne; -OH or NH3? [Select]
The Correct Answer and Explanation is:
Here are the correct answers and a detailed explanation for each question.
- A
- F
- ⁻NH₂
Explanation
1. The reaction of ethyne with ⁻OH:
The correct depiction is A. In an acid-base reaction, the base uses a lone pair of electrons to abstract a proton from the acid. The bond between the proton and the acid then breaks, with those electrons moving to the atom that lost the proton.
- In mechanism A, the curved arrow correctly shows a lone pair on the hydroxide ion (⁻OH, the base) attacking a proton on ethyne (the acid). The second arrow correctly shows the C-H bond breaking and the electrons moving onto the carbon, forming the acetylide anion (HC≡C⁻) and water (H₂O). Both the mechanism and the products are correct.
- Mechanism B is incorrect because the arrow originates from the C-H bond, which is not the source of electrons for forming the new O-H bond.
- Mechanism C is incorrect because the products are wrong; breaking the C-H bond cannot result in a positively charged carbon and a doubly negative oxygen.
2. The reaction of ethyne with ⁻NH₂:
The correct depiction is F. The same principles as the first reaction apply here. The amide ion (⁻NH₂) is the base, and ethyne is the acid.
- In mechanism F, the arrow from the lone pair on the nitrogen of the amide ion correctly attacks the proton on ethyne. The second arrow correctly shows the C-H bond electrons moving to the carbon, forming the acetylide anion (HC≡C⁻) and ammonia (NH₃). This represents the correct electron flow and products.
- Mechanism D shows incorrect products and arrow pushing.
- Mechanism E depicts a completely different, incorrect reaction type.
3. Which is a better base for removing a proton from ethyne?
The better base is ⁻NH₂. For an acid-base reaction to be favorable, the equilibrium must favor the products. This occurs when the products (the conjugate acid and conjugate base) are more stable (weaker) than the reactants (the original acid and base). A simple way to determine this is to compare the pKa of the acid on the reactant side with the pKa of the conjugate acid on the product side. The reaction will favor the side with the weaker acid (higher pKa).
- Reaction with ⁻OH:
HC≡CH (acid, pKa=25) + ⁻OH ⇌ HC≡C⁻ + H₂O (conjugate acid, pKa=15.7)
Since the pKa of the conjugate acid (water, 15.7) is much lower than the pKa of the starting acid (ethyne, 25), water is a much stronger acid. The equilibrium will lie far to the left, meaning this reaction does not proceed to a significant extent. - Reaction with ⁻NH₂:
HC≡CH (acid, pKa=25) + ⁻NH₂ ⇌ HC≡C⁻ + NH₃ (conjugate acid, pKa=36)
Here, the pKa of the conjugate acid (ammonia, 36) is higher than the pKa of ethyne (25). This means ammonia is a weaker acid than ethyne. Therefore, the equilibrium lies far to the right, strongly favoring the products. This makes the amide ion (⁻NH₂) an excellent base for deprotonating ethyne.
