Assign the characteristic functional groups with their IR peaks. 9-Fluorenol
The Correct Answer and Explanation is:
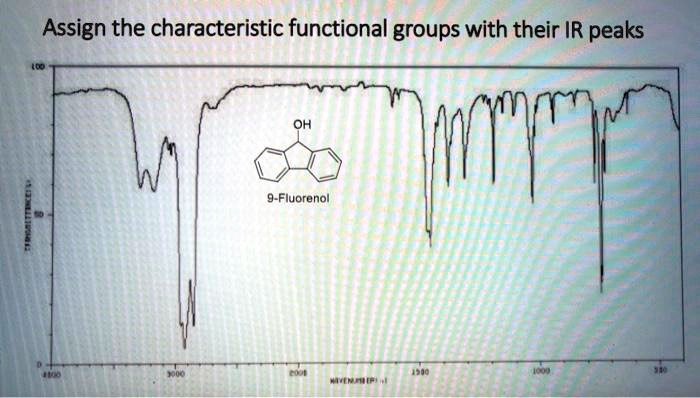
Here is the assignment of the characteristic functional groups in 9-Fluorenol with their corresponding peaks in the provided IR spectrum.
Correct Answer:
The IR spectrum of 9-Fluorenol can be analyzed by assigning the following absorption peaks to their respective functional groups:
- O-H Stretch (Alcohol): The broad, strong absorption band centered at approximately 3200 cm⁻¹.
- Aromatic C-H Stretch: The sharp peaks located just above 3000 cm⁻¹, in the range of 3020-3070 cm⁻¹.
- Aromatic C=C Stretch: The multiple sharp peaks in the region of 1450-1600 cm⁻¹.
- C-O Stretch (Secondary Alcohol): The strong, sharp peak at approximately 1015 cm⁻¹.
- Aromatic C-H Bend: The very strong, sharp peak at approximately 740 cm⁻¹.
Explanation
The IR spectrum of 9-Fluorenol displays characteristic absorptions that confirm its molecular structure, which includes a secondary alcohol and two aromatic rings. The analysis proceeds by identifying key vibrations from highest to lowest wavenumber.
- O-H Stretch (~3200 cm⁻¹): The most easily identifiable feature is the very broad and strong absorption band centered around 3200 cm⁻¹. This is the definitive signature for the O-H stretching vibration of an alcohol. The broadness of the peak is a direct result of intermolecular hydrogen bonding between the hydroxyl groups of different 9-Fluorenol molecules.
- C-H Stretches (~3050 cm⁻¹): The region around 3000 cm⁻¹ is crucial for distinguishing between different types of C-H bonds. The sharp absorptions seen slightly above 3000 cm⁻¹ (approx. 3020-3070 cm⁻¹) are characteristic of C-H stretches where the carbon is sp² hybridized, confirming the presence of aromatic rings. A weaker C-H stretch from the sp³-hybridized carbon at position 9 is expected just below 3000 cm⁻¹, although it may be less distinct.
- Aromatic C=C Stretches (1450-1600 cm⁻¹): The presence of several sharp peaks in the 1450-1600 cm⁻¹ region is indicative of carbon-carbon double bond (C=C) stretching within the aromatic rings. These “ring breathing” vibrations are a hallmark of aromatic compounds.
- C-O Stretch (~1015 cm⁻¹): In the fingerprint region, a strong and sharp absorption appears at about 1015 cm⁻¹. This peak is assigned to the C-O single bond stretching vibration. Its position is characteristic for a secondary alcohol, such as the one in 9-Fluorenol.
- Aromatic C-H Bending (~740 cm⁻¹): Finally, the very strong peak around 740 cm⁻¹ is due to the out-of-plane bending of the C-H bonds on the aromatic rings. The position of this powerful absorption is diagnostic for the substitution pattern; in this case, it is consistent with ortho-disubstituted rings.
