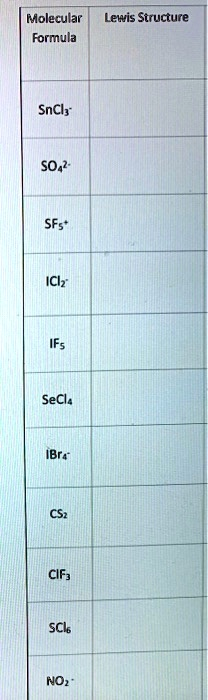
Molecular Formula and Lewis Structure table: SnCl3- SO4^2- SF5+ ICl2- IF5 SeCl4 IBr4- CS2 ClF3 SCl6 NO2-
The Correct Answer and Explanation is:
Of course. Here are the Lewis structures for the provided molecular formulas, along with a detailed explanation of how to determine them.
Explanation of Drawing Lewis Structures
A Lewis structure is a two-dimensional diagram that shows the bonding between atoms in a molecule and the lone pairs of electrons that may exist. The primary goal is to arrange the atoms and electrons so that each atom, if possible, achieves a stable electron configuration, usually a full octet (eight valence electrons).
The process for drawing a Lewis structure follows several key steps:
- Count Total Valence Electrons: Sum the valence electrons from all atoms in the molecule or ion. For a negative ion, add electrons equal to the charge. For a positive ion, subtract electrons.
- Determine the Central Atom: The central atom is typically the least electronegative element in the formula (excluding hydrogen). It is the atom that can form the most bonds.
- Draw a Skeleton Structure: Connect the other atoms (terminal atoms) to the central atom using single bonds. Each single bond represents two electrons.
- Distribute Remaining Electrons: Subtract the electrons used for bonding from the total valence electron count. Distribute the remaining electrons as lone pairs to the terminal atoms first, until they each have an octet.
- Place Leftover Electrons on the Central Atom: If any electrons are still left over after filling the octets of the terminal atoms, place them on the central atom, even if it exceeds an octet (this is common for elements in period 3 and below).
- Form Multiple Bonds if Necessary: If the central atom does not have an octet, move a lone pair from a terminal atom to form a double or triple bond with the central atom. This is done until the central atom’s octet is satisfied. The best structure is the one that minimizes formal charges on the atoms.
Correct Lewis Structures
Here are the descriptions for the Lewis structure of each molecule and ion listed:
- SnCl₃⁻: Tin (Sn) is the central atom. It is single-bonded to three Chlorine (Cl) atoms. Each Cl atom has three lone pairs. The Sn atom has one lone pair. The entire structure is enclosed in brackets with a -1 charge.
- SO₄²⁻: Sulfur (S) is the central atom. It forms two double bonds and two single bonds with the four Oxygen (O) atoms (this is one of several resonance structures that minimizes formal charge). The two double-bonded O atoms each have two lone pairs. The two single-bonded O atoms each have three lone pairs and a -1 formal charge. The structure is in brackets with a 2- charge.
- SF₅⁺: Sulfur (S) is the central atom, single-bonded to five Fluorine (F) atoms. Sulfur has an expanded octet and no lone pairs. Each F atom has three lone pairs. The structure is in brackets with a +1 charge.
- ICl₂⁻: Iodine (I) is the central atom, single-bonded to two Chlorine (Cl) atoms. Each Cl atom has three lone pairs. The central I atom has three lone pairs. The structure is in brackets with a -1 charge.
- IF₅: Iodine (I) is the central atom, single-bonded to five Fluorine (F) atoms. Each F atom has three lone pairs. The central I atom has one lone pair.
- SeCl₄: Selenium (Se) is the central atom, single-bonded to four Chlorine (Cl) atoms. Each Cl atom has three lone pairs. The central Se atom has one lone pair.
- IBr₄⁻: Iodine (I) is the central atom, single-bonded to four Bromine (Br) atoms. Each Br atom has three lone pairs. The central I atom has two lone pairs. The structure is in brackets with a -1 charge.
- CS₂: Carbon (C) is the central atom. It is double-bonded to two Sulfur (S) atoms. Each S atom has two lone pairs.
- ClF₃: Chlorine (Cl) is the central atom, single-bonded to three Fluorine (F) atoms. Each F atom has three lone pairs. The central Cl atom has two lone pairs.
- SCl₆: Sulfur (S) is the central atom, single-bonded to six Chlorine (Cl) atoms. Sulfur has an expanded octet and no lone pairs. Each Cl atom has three lone pairs.
- NO₂⁻: Nitrogen (N) is the central atom. It is single-bonded to one Oxygen (O) atom and double-bonded to the other O atom (this is a resonance structure). The single-bonded O has three lone pairs and a -1 formal charge. The double-bonded O has two lone pairs. The central N atom has one lone pair. The structure is in brackets with a -1 charge.
