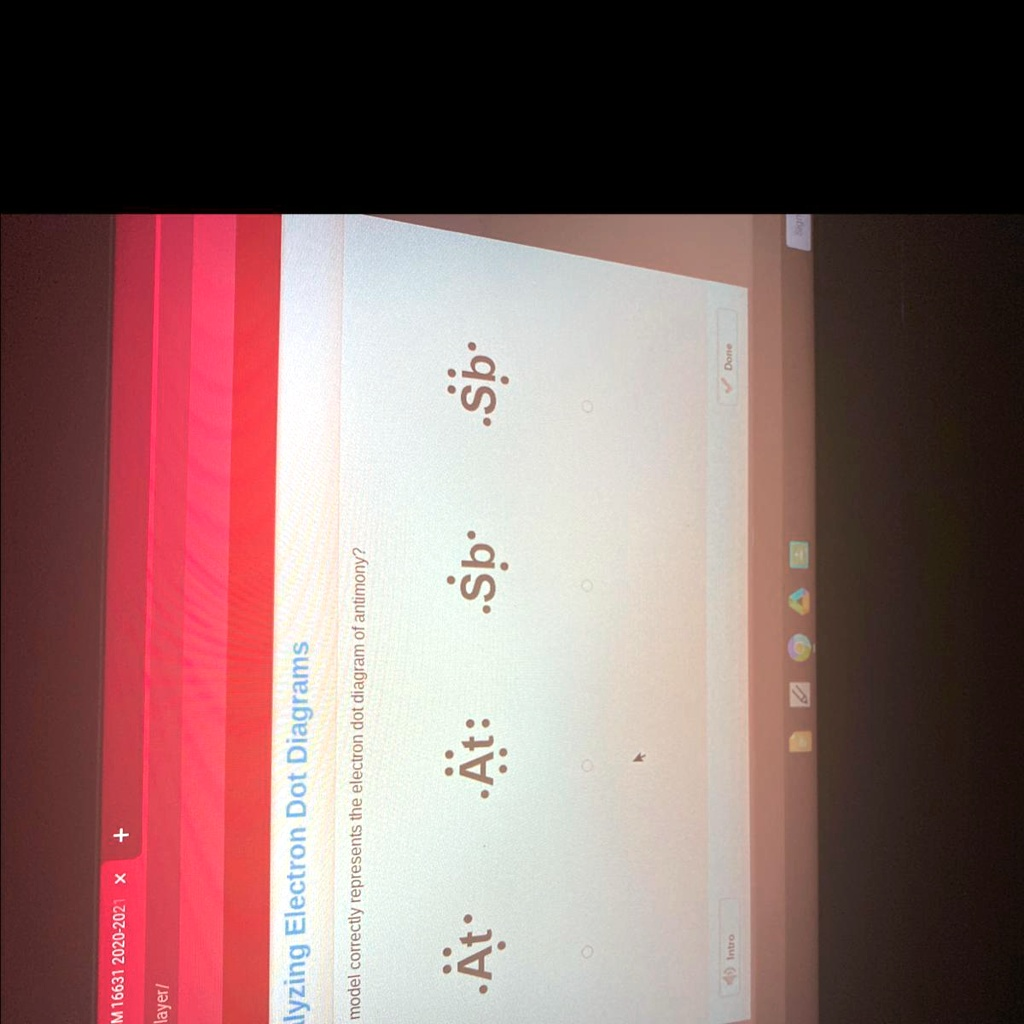
M16631 2020-2021 lyzing Electron Dot Diagrams model correctly represents the electron dot diagram of antimony
The Correct Answer and Explanation is:
The correct model is the second option for antimony, which shows the symbol Sb with a pair of dots on top and single dots on the other three sides.
An electron dot diagram, also known as a Lewis dot structure, is a visual representation of an atom’s valence electrons. Valence electrons are the electrons in the outermost energy shell of an atom, and they are the ones involved in chemical bonding. The diagram consists of the element’s chemical symbol surrounded by dots, where each dot represents one valence electron.
To determine the correct electron dot diagram for antimony (Sb), we must first find its number of valence electrons. This can be done by locating antimony on the periodic table. Antimony is element number 51 and resides in Group 15. For main group elements like antimony, the group number directly indicates the number of valence electrons. Therefore, all elements in Group 15, including antimony, have five valence electrons. This can also be confirmed by looking at antimony’s electron configuration, [Kr] 4d¹⁰ 5s² 5p³. The outermost shell is the fifth energy level (n=5), which contains two electrons in the 5s subshell and three electrons in the 5p subshell, for a total of 5 valence electrons.
When drawing the electron dot diagram, these five dots are placed around the chemical symbol, Sb. The standard convention is to place one dot on each of the four conceptual sides (top, bottom, left, and right) before creating any pairs. This practice reflects how electrons fill atomic orbitals according to Hund’s rule. For antimony’s five valence electrons, the first four are placed singly on each side. The fifth and final electron is then added to one of the sides to form a pair. This process results in a diagram with one pair of electrons and three unpaired, single electrons.
The correct model in the image accurately reflects this, showing the symbol Sb with one pair of dots and three single dots. The other diagram for antimony is incorrect because it only shows three dots, which would be appropriate for an element in Group 13, not Group 15.
