Determine which diagram best represents the ionic compound K,SO4 dissolved in water:
The Correct Answer and Explanation is:
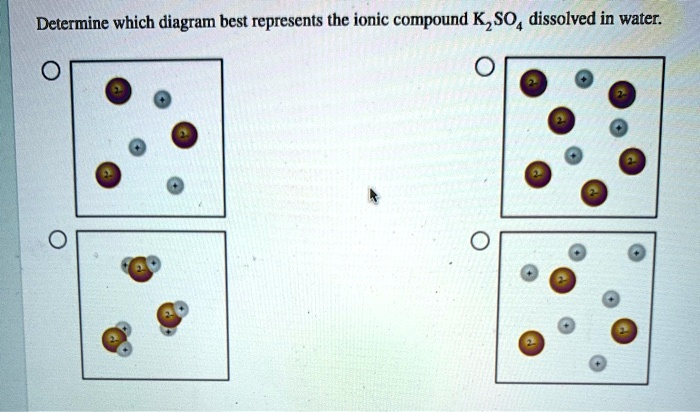
The correct answer is the diagram in the bottom right.
To determine the best representation of potassium sulfate (K₂SO₄) dissolved in water, we must first understand how this ionic compound behaves in an aqueous solution. Potassium sulfate is a strong electrolyte, which means it completely dissociates, or separates, into its constituent ions when dissolved in water.
The chemical formula K₂SO₄ provides two critical pieces of information. First, it identifies the ions involved. The compound is made of potassium (K) and the polyatomic ion sulfate (SO₄). Potassium is in Group 1 of the periodic table, so it forms a cation with a +1 charge (K⁺). The sulfate ion is a polyatomic anion with a charge of -2 (SO₄²⁻). In the diagrams, the small grey spheres with a single plus sign represent the K⁺ cations, and the larger brownish spheres with a “2-” represent the SO₄²⁻ anions.
Second, the subscripts in the formula indicate the ratio of these ions. The subscript ‘2’ next to K means that for every one formula unit of K₂SO₄ that dissolves, two potassium ions (2K⁺) and one sulfate ion (SO₄²⁻) are released into the solution. Therefore, the correct diagram must show a 2:1 ratio of K⁺ ions to SO₄²⁻ ions.
Let’s evaluate the options based on this 2:1 ratio. The top-left diagram shows a 1:1 ratio (three K⁺ and three SO₄²⁻), which is incorrect. The top-right diagram shows a 4:5 ratio, which is also incorrect.
Both the bottom-left and bottom-right diagrams show the correct 2:1 ratio, with six K⁺ ions and three SO₄²⁻ ions. However, the key difference is how the ions are distributed. Since K₂SO₄ dissociates completely, the ions should be separated from each other and dispersed randomly throughout the solution. The bottom-right diagram correctly depicts this, showing individual, free-moving ions. The bottom-left diagram incorrectly shows the ions still clustered together, which does not represent a fully dissolved and dissociated ionic compound.
