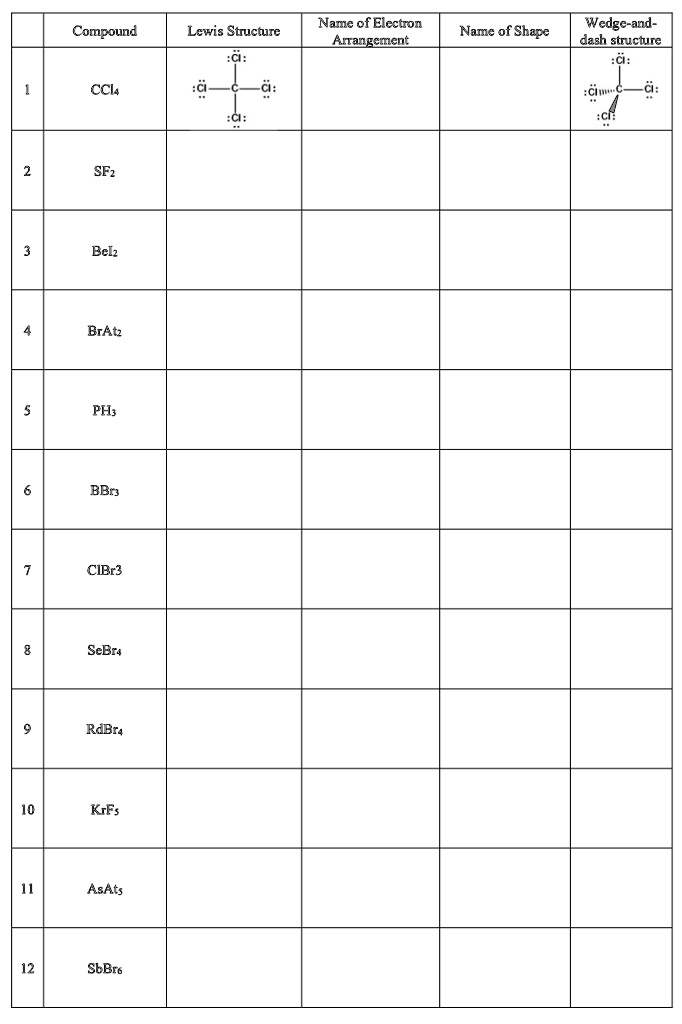
Name of Electron Arrangement Wedge-and-dash structure: Cl; Compound Lewis Structure Name of Shape CCl4 C2H6 SiH4 SF6 BeCl2 BrF3 PH3 BBr3 CIBr3 SCl2 RbBr KBr AsF5 SbF6
The Correct Answer and Explanation is:
Based on the provided table and the principles of VSEPR theory, here are the correct entries for the compound CCl₄ (carbon tetrachloride):
Name of Electron Arrangement: Tetrahedral
Name of Shape: Tetrahedral
Explanation
The names for both the electron arrangement and the molecular shape (geometry) are determined using the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory states that electron domains (which include bonding pairs and lone pairs) around a central atom will arrange themselves as far apart as possible to minimize electrostatic repulsion.
For the CCl₄ molecule:
- Identify the Central Atom and Valence Electrons: The central atom is Carbon (C) because it is the least electronegative. Carbon is in Group 14, so it has 4 valence electrons. Chlorine (Cl) is in Group 17 and has 7 valence electrons. With four chlorine atoms, the total number of valence electrons is 4 + (4 × 7) = 32.
- Determine Electron Domains: The Lewis structure, which is correctly drawn in the table, shows the central carbon atom forming single covalent bonds with four chlorine atoms. There are no lone pairs of electrons on the central carbon atom. Each of these four single bonds constitutes one electron domain. Therefore, the central carbon atom has a total of four electron domains.
- Name the Electron Arrangement: According to VSEPR theory, the arrangement that maximizes the distance between four electron domains is a tetrahedral arrangement. In this geometry, the electron domains point towards the vertices of a tetrahedron, with ideal bond angles of 109.5 degrees. This name describes the spatial arrangement of all electron domains (both bonding and non-bonding) around the central atom.
- Name the Molecular Shape: The molecular shape, or geometry, describes the arrangement of only the atoms in the molecule. It is determined by the positions of the bonding domains. Since CCl₄ has four bonding domains and zero lone pairs on the central atom (an AX₄ type molecule), the positions of the bonded chlorine atoms coincide perfectly with the vertices of the electron arrangement. Consequently, the molecular shape is also tetrahedral. The wedge-and-dash structure provided in the table visually confirms this three-dimensional tetrahedral arrangement of the atoms.
