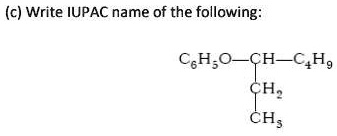
The Correct Answer and Explanation is:
3-Phenoxyheptane
Explanation:
To determine the IUPAC name for the given chemical structure, we follow a systematic approach based on IUPAC nomenclature rules for ethers.
- Identify the Functional Group: The compound contains an oxygen atom single bonded to two carbon-based groups (C₆H₅-O-R), which identifies it as an ether. One group is a phenyl group (C₆H₅), and the other is a complex alkyl group.
- Determine the Parent Alkane: For ethers, the IUPAC system names the compound as an alkoxy or aryloxy derivative of the larger or more complex hydrocarbon chain, which serves as the parent alkane. In this structure, the group attached to the oxygen is C₆H₅O- (a phenoxy group) and a branched alkyl chain. We must first identify the longest continuous carbon chain in this alkyl part. The central carbon (CH) is bonded to an ethyl group (-CH₂CH₃) and a butyl group (-C₄H₉, assumed to be a straight n-butyl chain). The longest continuous chain passes through this central carbon, combining the ethyl and butyl chains. This results in a chain of 2 (ethyl) + 1 (central CH) + 4 (butyl) = 7 carbon atoms. A seven-carbon alkane is called heptane. Thus, the parent alkane is heptane.
- Identify and Locate the Substituent: The substituent attached to the heptane parent chain is the phenoxy group (C₆H₅O-). We need to find its position (locant) on the heptane chain by numbering the carbons.
- Number the Parent Chain: The heptane chain must be numbered to give the substituent the lowest possible locant.
- Numbering from the end of the shorter side (the ethyl group): The phenoxy group is on carbon 3.
- Numbering from the end of the longer side (the butyl group): The phenoxy group would be on carbon 5.
- Assemble the Final Name: The name is constructed by putting the parts together in the order: locant, substituent name, and parent alkane name. This gives us 3-Phenoxyheptane.
