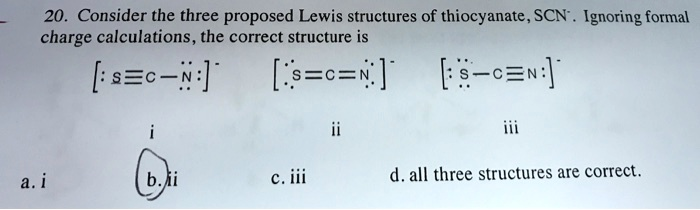
Consider the three proposed Lewis structures of thiocyanate, SCN. Ignoring formal charge calculations, the correct structure is [:S=C-N:]
[:S=C=N:]
[:S-C=N:]
i ii iii a. i b. ii c. iii d. all three structures are correct.
The Correct Answer and Explanation is:
The correct answer is b. ii.
Here is an explanation:
To determine the most appropriate Lewis structure for the thiocyanate ion (SCN⁻), we must consider a few key principles, even when asked to ignore formal charge calculations initially. The first steps are to count the total valence electrons and ensure each atom satisfies the octet rule.
- Valence Electron Count:
- Sulfur (S) is in Group 16, so it has 6 valence electrons.
- Carbon (C) is in Group 14, so it has 4 valence electrons.
- Nitrogen (N) is in Group 15, so it has 5 valence electrons.
- The ion has a -1 charge, which adds 1 electron.
- Total valence electrons = 6 + 4 + 5 + 1 = 16 electrons.
- Octet Rule Analysis:
- All three proposed structures (i, ii, and iii) correctly use all 16 valence electrons.
- Furthermore, in all three structures, each atom (Sulfur, Carbon, and Nitrogen) is surrounded by eight electrons, satisfying the octet rule.
Since all three options are technically valid Lewis structures that follow the octet rule, they are considered resonance structures. The question asks for “the correct structure,” which usually refers to the most stable or major resonance contributor. To determine this, we must evaluate the formal charges, despite the instruction to ignore them. The most stable structure is the one that minimizes formal charges and places any necessary negative charge on the most electronegative atom.
- Structure ii: [:S=C=N:]⁻
- Formal Charge on S = 6 – 4 – (4/2) = 0
- Formal Charge on C = 4 – 0 – (8/2) = 0
- Formal Charge on N = 5 – 4 – (4/2) = -1
- Structure iii: [:S-C≡N:]⁻
- Formal Charge on S = 6 – 6 – (2/2) = -1
- Formal Charge on C = 4 – 0 – (8/2) = 0
- Formal Charge on N = 5 – 2 – (6/2) = 0
Both structures ii and iii have minimal formal charges. However, we must consider electronegativity. Nitrogen is more electronegative than Sulfur. Therefore, the structure that places the negative formal charge on the more electronegative Nitrogen atom (Structure ii) is the most stable and significant contributor to the resonance hybrid. Structure i is the least stable as it results in a +1 charge on sulfur and a -2 charge on nitrogen.
Thus, structure ii is considered the best and most correct representation of the thiocyanate ion.
