Give the expected product from the following reaction.
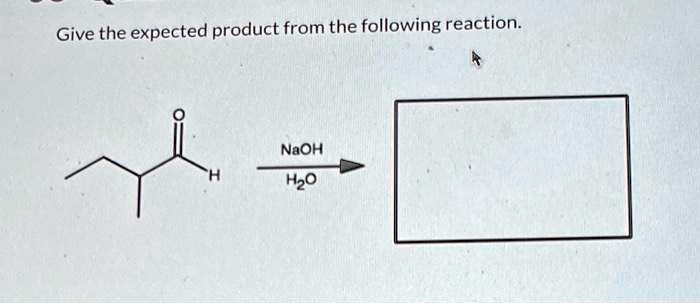
The Correct Answer and Explanation is:
to the aldehyde group. This specific product is called an “aldol” (aldehyde + alcohol).
The product formed is 3-hydroxy-2-ethyl-2,4-dimethylhexanal.
Typically, an aldol addition can be followed by a dehydration (or condensation) step to form a more stable α,β-unsaturated aldehyde, especially with heat or strong base. This dehydration requires the removal of a hydrogen atom from the alpha-carbon of the aldol product. However, in this specific case, the alpha-carbon of the resulting aldol product (3-hydroxy-2-ethyl-2,4-dimethylhexanal) is a quaternary carbon. It is bonded to the carbonyl group, the β-carbon, a methyl group, and an ethyl group. Since it has no hydrogen atoms attached, the dehydration step cannot occur. Therefore, the reaction stops at the aldol addition product, which is the final expected product.
