NaBrO HBr OH H2O MeOH HBr OH SH NaSH NaHO2
The Correct Answer and Explanation is:
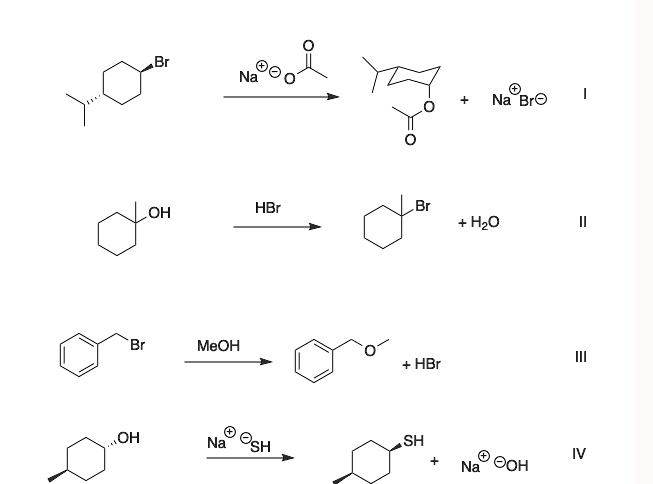
The correct answer is that reactions I, II, and III are SN1 reactions.
Here is a detailed explanation for each reaction:
Reaction I: This reaction involves a secondary alkyl halide, trans-1-bromo-4-isopropylcyclohexane, reacting with sodium acetate, a weak nucleophile. The SN1 mechanism is favored over SN2 for several reasons. The substrate’s most stable chair conformation places both the bulky isopropyl group and the bromine atom in equatorial positions. For an SN2 reaction to occur, the acetate nucleophile would need to perform a backside attack from a sterically hindered axial position. This makes the SN2 pathway slow. Instead, the reaction is more likely to proceed via the SN1 mechanism, where the C-Br bond breaks first to form a secondary carbocation intermediate, which is then attacked by the acetate nucleophile.
Reaction II: This is a classic example of an SN1 reaction. The substrate is a tertiary alcohol, 1-methylcyclohexan-1-ol. SN2 reactions are impossible at sterically crowded tertiary centers. The reaction proceeds in two main steps: first, the hydroxyl (-OH) group is protonated by the strong acid (HBr) to form a good leaving group, water (-OH2+). Second, the water molecule departs, generating a stable tertiary carbocation. Finally, the bromide ion (Br-) acts as a nucleophile and attacks the carbocation to form the final product. The formation of a stable carbocation intermediate is the defining characteristic of an SN1 reaction.
Reaction III: This reaction shows a benzylic halide (benzyl bromide) undergoing solvolysis with methanol, which is a weak nucleophile and a polar protic solvent. Although the substrate is technically a primary halide, the SN1 pathway is highly favored. This is because the departure of the bromide leaving group forms a benzylic carbocation. This carbocation is significantly stabilized by resonance, as the positive charge can be delocalized over the adjacent benzene ring. The combination of a substrate that forms a highly stable carbocation and a weak nucleophile makes this a textbook SN1 reaction.
Reaction IV: This reaction is not an SN1 reaction; it displays the characteristics of an SN2 reaction. The substrate reacts with sodium hydrosulfide (NaSH), where the hydrosulfide ion (SH-) is a strong nucleophile. Most importantly, the reaction shows a clear inversion of stereochemistry: the trans starting material yields a cis product. This stereospecific inversion is the hallmark of the SN2 mechanism, where the nucleophile attacks from the side opposite the leaving group in a single, concerted step. An SN1 reaction would have proceeded through a planar carbocation, leading to a mixture of products (both retention and inversion), not a single inverted product.
