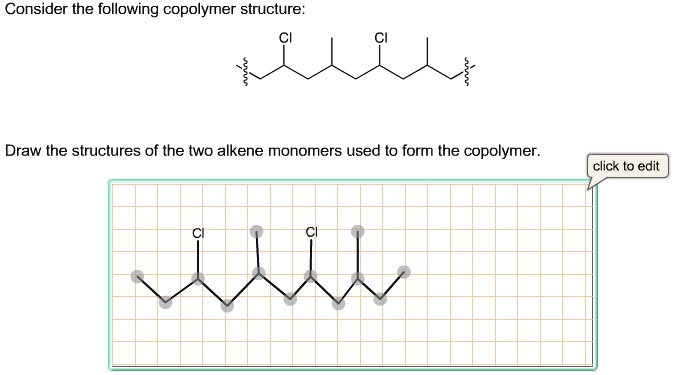
Consider the following copolymer structure: CI CI Draw the structures of the two alkene monomers used to form the copolymer. CI CI
The Correct Answer and Explanation is:
The correct answer is the two alkene monomers: chloroethene and propene.
Chloroethene (Vinyl chloride)Generated code
H Cl
\ /
C=C
/ \
H HCondensed formula: CH2=CHCl
Propene (Propylene)Generated code
H CH3
\ /
C=C
/ \
H HCondensed formula: CH2=CHCH3
Explanation
The provided chemical structure is a copolymer, which is a polymer derived from two or more different types of monomer units. This specific polymer is formed through addition polymerization, a process where alkene monomers add to one another in such a way that the polymer contains all the atoms of the monomer units. During this reaction, the pi (π) bond of the carbon-carbon double bond in each alkene monomer breaks, and new sigma (σ) bonds form to create the long, saturated backbone of the polymer.
To determine the original monomers, we must first analyze the repeating structure of the copolymer chain. The given structure is:
…-CH(Cl)-CH2-CH(CH3)-CH2-CH(Cl)-CH2-CH(CH3)-CH2-…
By examining this structure, we can identify a distinct, alternating pattern. The polymer is built from two different two-carbon units that repeat. The overall four-carbon repeating unit of this alternating copolymer is [-CH(Cl)-CH2-CH(CH3)-CH2-]. This unit is composed of two smaller monomeric fragments linked together.
To find the structures of the original alkene monomers, we can mentally reverse the polymerization process. This involves breaking the sigma bonds in the backbone that connect the monomer units and reforming the carbon-carbon double bonds within each unit.
- The first two-carbon fragment in the repeating unit is -CH(Cl)-CH2-. If we re-form the double bond between these two carbon atoms, we get the alkene CH2=CHCl. This molecule is named chloroethene, commonly known as vinyl chloride.
- The second two-carbon fragment in the repeating unit is -CH(CH3)-CH2-. Re-forming the double bond between its two backbone carbons gives the alkene CH2=CH(CH3). This molecule is named propene, commonly known as propylene.
Therefore, the two alkene monomers used to form the given copolymer are chloroethene and propene.
