Benzophenone starting material INFRARED SPECTRUM 0.8 L 0.6 1 0.2 3000 2000 Wavenumber (cm-1) 1000
The Correct Answer and Explanation is:
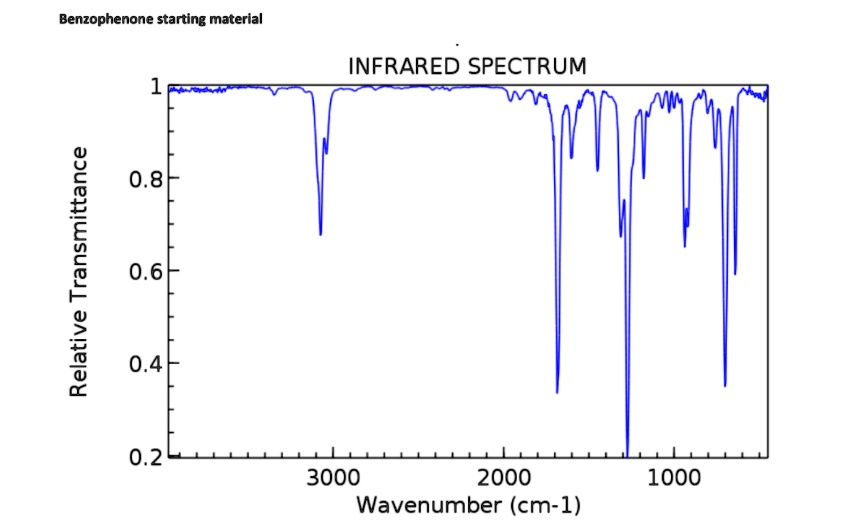
Correct Answer: The infrared spectrum confirms the structure of benzophenone. Key absorptions include aromatic C-H stretches above 3000 cm⁻¹, a strong carbonyl (C=O) stretch at approximately 1660 cm⁻¹, aromatic C=C stretches between 1450-1600 cm⁻¹, and C-H bending vibrations below 900 cm⁻¹.
Explanation:
The provided infrared (IR) spectrum is consistent with the molecular structure of benzophenone, which consists of a central carbonyl group bonded to two phenyl rings. The analysis of specific absorption peaks confirms the presence of the key functional groups.
The region just above 3000 cm⁻¹ shows several sharp peaks, which are characteristic of the C-H stretching vibrations of sp2-hybridized carbons in an aromatic ring. The absence of peaks in the 2850-3000 cm⁻¹ range confirms the lack of aliphatic sp3-hybridized C-H bonds.
The most diagnostic and intense peak in this spectrum is the sharp absorption at approximately 1660 cm⁻¹. This signal is characteristic of a carbonyl (C=O) stretching vibration. The position of this peak is lower than that of a typical saturated ketone (around 1715 cm⁻¹) due to conjugation. The delocalization of electrons between the carbonyl group and the two attached aromatic rings weakens the C=O double bond, requiring less energy to stretch and thus lowering its absorption frequency. This is a hallmark of an aromatic ketone.
Additionally, the spectrum displays several absorptions in the 1450-1600 cm⁻¹ range. These peaks are attributed to the C=C stretching vibrations within the aromatic rings themselves, further supporting the presence of the phenyl groups.
Finally, the fingerprint region below 1500 cm⁻¹ provides more structural information. The strong, sharp peaks observed around 700 cm⁻¹ and 750 cm⁻¹ are typical for the out-of-plane C-H bending vibrations of a monosubstituted benzene ring. The presence of these absorptions strongly suggests this substitution pattern. The lack of a broad peak in the 3200-3600 cm⁻¹ region confirms the absence of any hydroxyl (O-H) groups. In summary, all the observed spectral features align perfectly with the structure of benzophenone.
