Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table
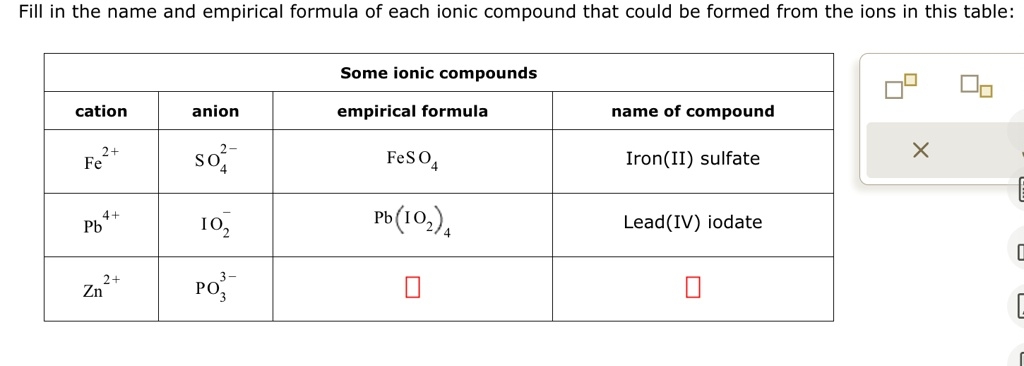
The Correct Answer and Explanation is:
Empirical Formula: Zn₃(PO₃)₂
Name of Compound: Zinc phosphite
Explanation:
To determine the empirical formula and name of the ionic compound formed from the zinc cation (Zn²⁺) and the phosphite anion (PO₃³⁻), we follow two main steps: balancing the charges for the formula and applying IUPAC naming rules.
1. Determining the Empirical Formula:
The primary rule for forming an ionic compound is that the overall charge must be neutral. This means the total positive charge from the cations must equal the total negative charge from the anions.
- Cation: Zinc, Zn²⁺ (charge of +2)
- Anion: Phosphite, PO₃³⁻ (charge of -3)
To find the simplest whole-number ratio of ions that results in a neutral compound, we can find the least common multiple (LCM) of the magnitudes of the charges (2 and 3). The LCM of 2 and 3 is 6.
- To get a total positive charge of +6, we need three zinc ions: 3 × (+2) = +6.
- To get a total negative charge of -6, we need two phosphite ions: 2 × (-3) = -6.
Thus, the ratio of Zn²⁺ to PO₃³⁻ is 3:2. In the chemical formula, the subscript for each ion corresponds to the number of ions needed for neutrality. When a polyatomic ion (like phosphite) requires a subscript greater than one, it is enclosed in parentheses. This leads to the empirical formula: Zn₃(PO₃)₂.
2. Naming the Compound:
The name of an ionic compound is formed by combining the name of the cation and the name of the anion.
- Cation Name: The cation is Zn²⁺. Zinc is a transition metal that typically only forms a +2 ion. Unlike metals with variable charges like iron (Fe) or lead (Pb) that require a Roman numeral to indicate the charge (e.g., Iron(II)), zinc’s charge is considered fixed. Therefore, the Roman numeral is omitted, and the cation is simply named zinc.
- Anion Name: The anion is PO₃³⁻. This is a polyatomic ion named phosphite.
Combining the cation and anion names gives the full name of the compound: Zinc phosphite.
