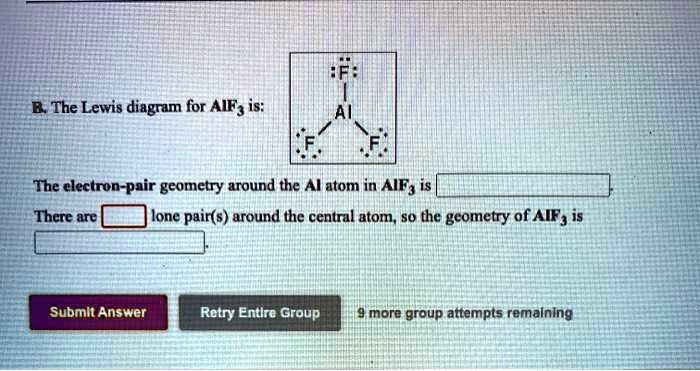
The Lewis diagram for AlF3 is: The electron-pair geometry around the Al atom in AlF3 is trigonal planar. There are no lone pairs around the central atom, so the geometry of AlF3 is trigonal planar.
The Correct Answer and Explanation is:
Based on the provided Lewis structure for Aluminum Trifluoride (AlF₃), here are the correct answers:
The electron-pair geometry around the Al atom in AlF₃ is trigonal planar.
There are 0 lone pair(s) around the central atom, so the geometry of AlF₃ is trigonal planar.
Explanation
The geometry of a molecule is determined by the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory states that electron pairs in the valence shell of a central atom repel each other and will arrange themselves to be as far apart as possible, which minimizes repulsion and determines the molecule’s shape.
Electron-Pair Geometry
To find the electron-pair geometry, we first identify the central atom, which is aluminum (Al) in AlF₃. Next, we count the number of electron domains around it. An electron domain is any region of high electron density, which includes single bonds, double bonds, triple bonds, and lone pairs. In the Lewis structure of AlF₃, the aluminum atom is bonded to three fluorine atoms through single bonds. There are no lone pairs of electrons on the central aluminum atom.
Therefore, the aluminum atom has three electron domains (three single bonds + zero lone pairs). According to VSEPR theory, three electron domains will arrange themselves in a flat triangle around the central atom to maximize their separation. This arrangement is called a trigonal planar electron-pair geometry, with ideal bond angles of 120 degrees.
Molecular Geometry
The molecular geometry describes the arrangement of only the atoms in the molecule. It is derived from the electron-pair geometry but considers the effect of lone pairs. To determine the molecular geometry, we look at the number of bonding domains and lone pair domains separately.
For AlF₃, we have:
- Bonding domains: 3 (from the three Al-F bonds)
- Lone pair domains: 0
When there are no lone pairs on the central atom, the repulsion forces are evenly distributed among the bonding pairs. As a result, the arrangement of the atoms is identical to the arrangement of the electron pairs. Since the electron-pair geometry is trigonal planar and there are zero lone pairs, the molecular geometry of AlF₃ is also trigonal planar.
