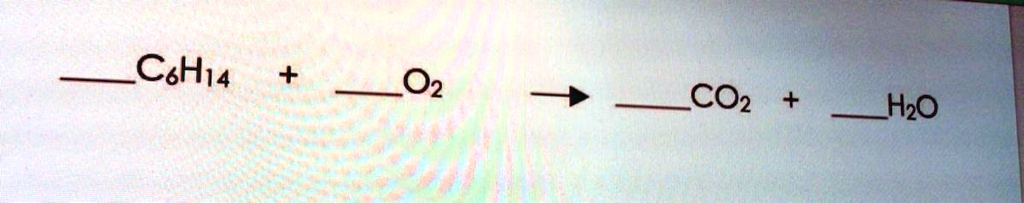
The Correct Answer and Explanation is:
Here is the correctly balanced chemical equation:
2 C₆H₁₄ + 19 O₂ → 12 CO₂ + 14 H₂O
This equation represents the complete combustion of hexane (C₆H₁₄), a type of hydrocarbon. Balancing a chemical equation is essential as it must adhere to the law of conservation of mass, which states that atoms are neither created nor destroyed during a chemical reaction. Therefore, the number of atoms for each element must be identical on both the reactant (left) and product (right) sides of the equation.
The process to achieve this balance involves adjusting the stoichiometric coefficients, which are the numbers placed in front of each chemical formula.
- Balance Carbon (C): We begin with the most complex molecule, C₆H₁₄. There are 6 carbon atoms on the reactant side. To balance this, we place a coefficient of 6 in front of CO₂ on the product side, giving us 6 carbon atoms there as well.
C₆H₁₄ + O₂ → 6 CO₂ + H₂O - Balance Hydrogen (H): Next, we balance hydrogen. The reactant C₆H₁₄ contains 14 hydrogen atoms. On the product side, each water molecule (H₂O) has 2 hydrogen atoms. To get 14 hydrogen atoms, we need 7 water molecules (7 x 2 = 14). So, we place a 7 in front of H₂O.
C₆H₁₄ + O₂ → 6 CO₂ + 7 H₂O - Balance Oxygen (O): Now, we balance the oxygen atoms. On the product side, we have 6 CO₂ molecules, which contain 12 oxygen atoms (6 x 2), and 7 H₂O molecules, which contain 7 oxygen atoms (7 x 1). This gives a total of 19 oxygen atoms on the product side. To get 19 oxygen atoms on the reactant side from O₂, we would need a coefficient of 19/2, since oxygen exists as a diatomic molecule.
C₆H₁₄ + (19/2) O₂ → 6 CO₂ + 7 H₂O - Use Whole Numbers: By convention, chemical equations use whole number coefficients. To eliminate the fraction, we multiply every coefficient in the entire equation by the denominator, which is 2. This gives us the final, correctly balanced equation:
2 C₆H₁₄ + 19 O₂ → 12 CO₂ + 14 H₂O
