Discussion Worksheet 1. What is the relationship between molar mass and boiling point? Offer an explanation for this relationship based on intermolecular forces. The higher the boiling point, the stronger attractions between molecules, therefore, the molar mass & boiling points have a very significant strong relationship. 2. Which compound would you expect to have a higher boiling point? Use intermolecular forces to explain your answer. A H Cl B Cl H H H H C C H H Cl Cl H Cl The first compound would have a higher boiling point, due to it having a stronger molecular attraction. 3. Which of the substances below can exhibit dipole-dipole interactions between molecules of the same type (e.g., Cl2 with Cl2)? Select all that apply. a. Cl2 b. F2 c. ClF d. NF3 4. Which of the substances below can exhibit hydrogen bonding between molecules of the same type? Select all that apply. a. n-butanol: CH3CH2CH2CH2OH b. t-butanol: (CH3)3OH c. ethylmethylether: CH3CH2OCH3 d. n-pentane: CH3CH2CH2CH2CH3
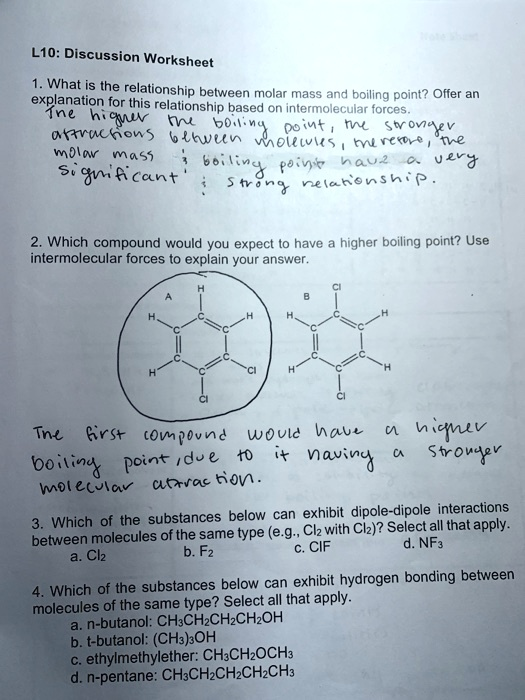
The Correct Answer and Explanation is:
Here are the correct answers and detailed explanations for the worksheet questions.
1. What is the relationship between molar mass and boiling point? Offer an explanation for this relationship based on intermolecular forces.
The general relationship is that for molecules with similar structures and types of intermolecular forces, the boiling point increases as the molar mass increases.
The explanation is based on London dispersion forces (LDFs), which are present in all molecules. LDFs are temporary attractions that occur because of the constant motion of electrons, creating instantaneous, temporary dipoles. A molecule with a larger molar mass typically has more electrons. This larger cloud of electrons is more easily distorted, or “polarizable,” which allows for the formation of larger temporary dipoles. These larger dipoles result in stronger LDFs between molecules. Since more energy is required to overcome these stronger forces, the substance will have a higher boiling point.
2. Which compound would you expect to have a higher boiling point? Use intermolecular forces to explain your answer.
Compound B would have a higher boiling point.
Both compounds, A (1,4-dichlorobenzene) and B (1,2-dichlorobenzene), are isomers and have the same molar mass. Therefore, we must compare the strength of their other intermolecular forces. The key difference is their molecular polarity. In compound A, the two polar C-Cl bonds are positioned directly opposite each other, causing their individual bond dipoles to cancel out. This makes compound A a nonpolar molecule. In compound B, the C-Cl bonds are adjacent, and their dipoles do not cancel, resulting in a net molecular dipole. This makes compound B a polar molecule.
Since compound B is polar, it experiences both London dispersion forces and stronger dipole-dipole interactions. Compound A is nonpolar and only has London dispersion forces. Because compound B has stronger overall intermolecular forces, more energy is needed to separate its molecules, giving it a higher boiling point.
3. Which of the substances below can exhibit dipole-dipole interactions between molecules of the same type? Select all that apply.
Correct Answers: c. ClF and d. NF₃
Dipole-dipole interactions occur between polar molecules.
- a. Cl₂ and b. F₂ are nonpolar because they are diatomic molecules made of identical atoms, so there is no difference in electronegativity.
- c. ClF is a polar molecule because fluorine is more electronegative than chlorine, creating a permanent dipole.
- d. NF₃ is a polar molecule. Although the N-F bonds are arranged in a trigonal pyramidal shape, the geometry is asymmetrical due to the lone pair on the nitrogen atom, so the bond dipoles do not cancel out.
4. Which of the substances below can exhibit hydrogen bonding between molecules of the same type? Select all that apply.
Correct Answers: a. n-butanol and b. t-butanol
Hydrogen bonding occurs when a hydrogen atom is directly bonded to a highly electronegative atom (nitrogen, oxygen, or fluorine).
- a. n-butanol (CH₃CH₂CH₂CH₂OH) and b. t-butanol ((CH₃)₃COH) both contain an -OH group, where a hydrogen atom is bonded to an oxygen atom. They can therefore form hydrogen bonds with each other.
- c. ethylmethylether (CH₃CH₂OCH₃) has an oxygen atom, but all its hydrogens are bonded to carbon atoms, not oxygen. Thus, it cannot hydrogen bond with itself.
- d. n-pentane is a hydrocarbon containing only C-H and C-C bonds and cannot form hydrogen bonds.
