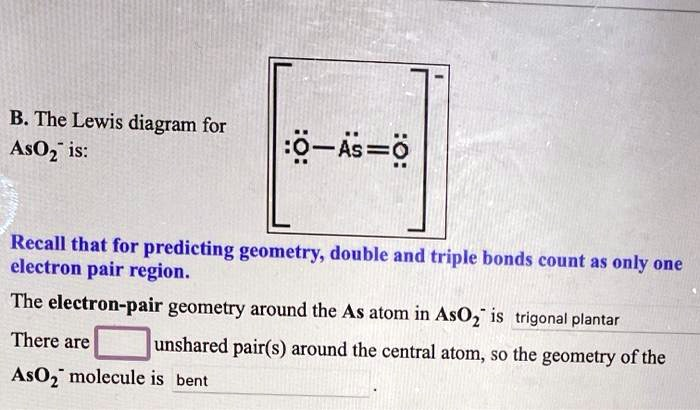
The Lewis diagram for AsO2- is: :8~4s=8 Recall that for predicting geometry, double and triple bonds electron pair region count as only one. The electron-pair geometry around the As atom in AsO2- is trigonal planar. There is an unshared pair around the central atom, so the geometry of the AsO2- molecule is bent.
The Correct Answer and Explanation is:
From the image, the molecule in question is AsO2-, the arsenite ion. The Lewis diagram for AsO2- shows that the arsenic (As) atom is in the center, bonded to two oxygen (O) atoms via double bonds, with a lone pair of electrons on the arsenic atom.
Explanation:
- Electron-pair geometry:
The electron-pair geometry is determined by the number of bonding and lone pairs of electrons around the central atom. Here, arsenic (As) has two double bonds (each with an oxygen atom) and one lone pair of electrons, giving a total of three regions of electron density around it. According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, when there are three regions of electron density, the geometry is trigonal planar. - Molecular geometry:
The molecular geometry is influenced by the actual positions of the atoms, not just the electron pairs. Since there is a lone pair of electrons on the central arsenic atom, this lone pair exerts repulsion, pushing the two oxygen atoms closer together. This causes the geometry to be bent rather than trigonal planar. The lone pair is not counted in the molecular geometry, only the positions of the oxygen atoms, resulting in a bent shape.
Correct Answer:
- The electron-pair geometry around the arsenic (As) atom in AsO2- is trigonal planar.
- There is one lone pair around the central arsenic atom.
- The molecular geometry of the AsO2- molecule is bent.
This conclusion is based on the VSEPR theory, which helps explain the molecular shape based on electron-pair repulsion. The lone pair reduces the available space for bonding pairs, resulting in the bent shape.
