??? LOD ???/% 50 0 4000 3000 2000 1500 1000 500 ??/cm?¹ 3326 23 3164 22 3035 39 2964 10 2924 4 2854 10 2720 68 2696 70 2669 68 2590 70 1666 10 1611 18 1555 14 1516 22 1608 14 1444 15 1376 29 1328 37 1281 70 1261 18 1244 27 1228 26 1173 50 1109 66 1016 68 970 66 839 35 809 32 797 47 716 49 687 46 626 60 605 64 521 44 504 44 HO-NH-C-CH? O
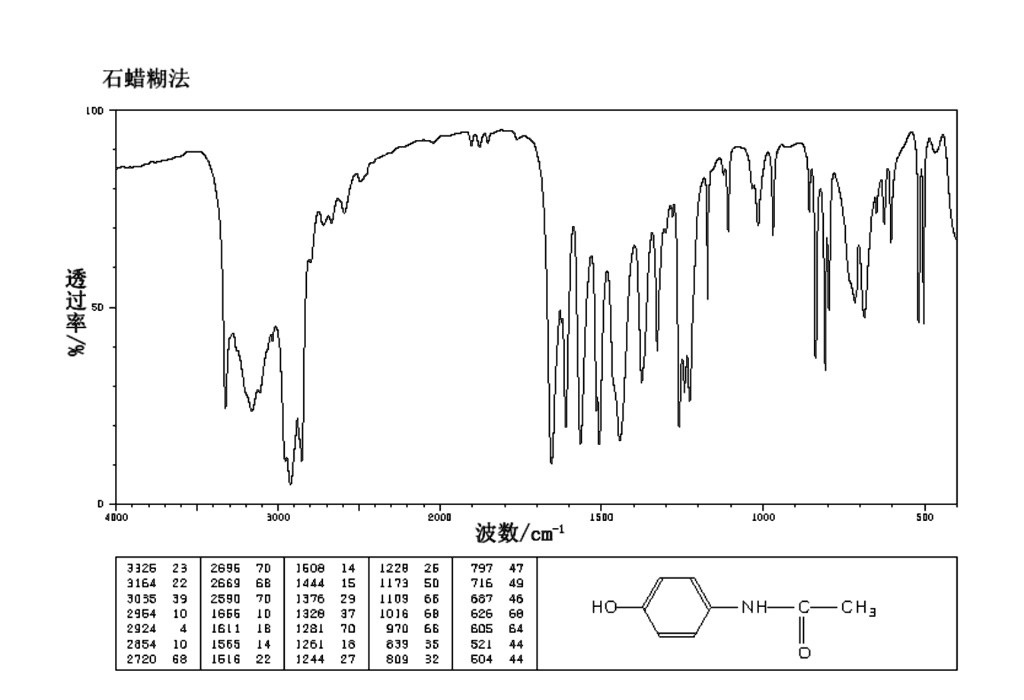
The Correct Answer and Explanation is:
Correct Answer: N-(4-hydroxyphenyl)acetamide
Explanation:
The image displays the Infrared (IR) spectrum of the compound N-(4-hydroxyphenyl)acetamide, also commonly known as paracetamol or acetaminophen, whose chemical structure is shown in the bottom right corner. The analysis of the absorption bands in the spectrum confirms the presence of all the key functional groups in this molecule.
- **O-H and N-H Stretching Region broadness is due to intermolecular hydrogen bonding, a characteristic feature of molecules containing these functional groups in the solid state.
(3500-3100 cm⁻¹):** The spectrum shows a broad absorption band centered around 3A very strong and sharp absorption is observed at 1666 cm⁻¹. This is the Amide I band,164 cm⁻¹ and a sharper peak at 3326 cm⁻¹. The broadness is characteristic of the which is characteristic of the carbonyl (C=O) stretching vibration within the secondary amide group. Its high intensity and specific hydrogen-bonded O-H stretching vibration of the phenolic group. The sharper peak at 3326 cm⁻¹ is attributed position are strong indicators of this functional group.
Further confirmation of the amide is provided by the Amide II band at to the N-H stretching vibration of the secondary amide group.
- C-H Stretching Region (3 1555 cm⁻¹. This absorption results from a combination of N-H in-plane bending and C-N stretching100-2800 cm⁻¹): The peak at 3035 cm⁻¹ is characteristic vibrations. The presence of both strong Amide I and Amide II bands is definitive evidence for a secondary amide.
of aromatic C-H stretching from the benzene ring. The weaker absorptions below 3000 cm⁻¹The absorptions for the aromatic ring are also clearly visible. The peak at 3035 cm⁻¹ corresponds (e.g., 2924 cm⁻¹) correspond to the aliphatic C-H stretching of the methyl (- to aromatic C-H stretching. The peaks at 1611 cm⁻¹ and 1516 cm⁻CH₃) group. The title “石蜡糊法” (Nujol mull method) indicates that some of¹ are due to C=C stretching vibrations within the benzene ring. Crucially, the strong peak at 839 cm⁻¹ is characteristic of the C-H out-of-plane bending for a 1,4-disub these C-H signals may overlap with those from the Nujol (mineral oil) mulling agent.
3.stituted (para) benzene ring, which matches the substitution pattern of paracetamol.
Finally, the title “石蜡糊 Amide Bands (1700-1500 cm⁻¹): This region contains the most characteristic signals for the amide group. The very strong, sharp absorption at 1666 cm⁻¹ is the法” indicates the sample was prepared using a Nujol mull. Nujol is a mineral oil, and its characteristic C-H stretching peaks (around 2924 cm⁻¹ and 2854 cm⁻¹) and bending Amide I band, which is primarily due to the C=O carbonyl stretching vibration. The strong absorptions at 15 peaks (around 1444 cm⁻¹ and 1376 cm⁻¹) are present in the spectrum,65 cm⁻¹ and 1516 cm⁻¹ correspond to the Amide II band, which arises from a combination of N-H bending and C-N stretching.
- Aromatic C=C Stretching (1610-1450 cm⁻¹): The peaks at 1611 cm⁻¹ and 1608 cm⁻¹ are due to the C=C stretching vibrations within the aromatic ring.
- Fingerprint Region (< 1500 cm⁻¹): This complex region contains many significant peaks. Notably which is expected for this technique. The combination of all these spectral features provides a conclusive identification of the compound as N-(4-hydroxyphenyl)acetamide., the series of strong peaks between 1261 cm⁻¹ and 1228 cm⁻¹ is characteristic of the C-O stretching vibration of the phenol group. Furthermore, the strong peak at 839 cm⁻¹ is a key diagnostic band for the out-of-plane C-H bending of a 1,4-disub
