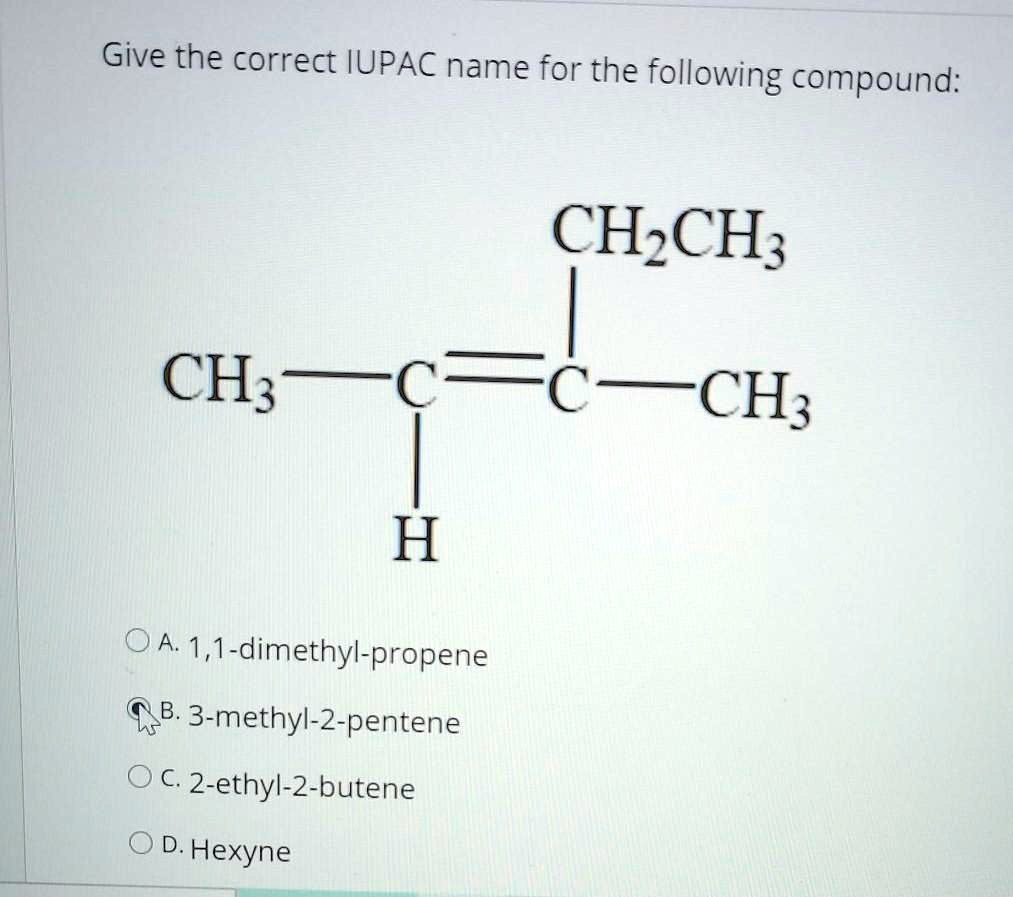
Give the correct IUPAC name for the following compound: CH3CH=CH2 CH3CH2CH=CH2 A. 1,1-dimethylpropene B. 3-methyl-2-pentene C. 2-ethyl-2-butene D. Hexyne FC=CH3
The Correct Answer and Explanation is:
The correct IUPAC name for the given compound is B. 3-methyl-2-pentene.
Explanation:
To determine the correct IUPAC name for an alkene, we follow a set of systematic rules:
- Identify the Parent Chain: The first step is to find the longest continuous chain of carbon atoms that includes the double bond. In the provided structure, CH3–CH=C(CH3)(CH2CH3), the longest carbon chain that contains the C=C double bond is five carbons long (CH3–CH=C–CH2–CH3). Therefore, the parent name is derived from “pentane,” and because it’s an alkene (contains a double bond), the suffix becomes “-ene.” The parent name is pentene.
- Number the Parent Chain: The chain must be numbered to give the carbon atoms of the double bond the lowest possible numbers.
- Numbering from left to right: C1H3–C2H=C3(CH3)–C4H2–C5H3. The double bond starts at carbon 2.
- Numbering from right to left: C5H3–C4H2–C3(CH3)=C2H–C1H3. The double bond would start at carbon 3.
Since 2 is lower than 3, we number the chain from left to right. This makes the compound a 2-pentene.
- Identify and Name Substituents: Now, we identify any groups attached to the parent chain. Using the correct numbering (from left to right), there is a methyl group (–CH3) attached to carbon 3. So, the substituent is named 3-methyl.
- Assemble the Full Name: Finally, we combine the parts into a single name. The substituent name and its position are placed before the parent chain name. This gives us the final IUPAC name: 3-methyl-2-pentene.
Analysis of Other Options:
- A. 1,1-dimethyl-propene: This name is invalid. The parent chain is pentene, not propene.
- C. 2-ethyl-2-butene: This name incorrectly identifies the parent chain. While a four-carbon chain (butene) exists, the IUPAC rules require using the longest possible chain that includes the double bond, which is five carbons.
- D. Hexyne: This is incorrect. The molecule has a double bond (it’s an alkene, not an alkyne with a triple bond) and its longest chain has five carbons, not six (hex-).
